
You complete your downstream analyses, pull up the data on your screen, and your heart sinks. Your baseline values are well above average, obscuring any significant differences between your controls and your variables. You vaguely remember that your cells may have looked a little rough by the end of the experiment, but you figured that was a result of the test solutions you were subjecting them to. Trying not to panic, you double-checked your calculations and dilutions with three colleagues, and each of them confirmed they were done correctly. Seemingly everything checked out, and you were exact on your time points.
You were entirely prepared for this experiment and executed it without issue. So, what went wrong?
Experimental success begins with happy cells!
Find out 8 ways to optimize your cell cultures:
- What’s on the menu?
- Do your cells feel comfortable?
- How friendly are your cells?
- How adherent are your cells?
- What home do your cells prefer?
- What volumes are being transferred?
- Are your cells hosting an uninvited guest?
- We are all human, but we don’t have to measure inaccurately like one!
-
What’s on the menu?
While all culture medium contains the essentials for sustaining healthy cells, every cell type will thrive best when they are provided their ideal combinations and concentrations of key nutrients and supplements. DMEM and RPMI are widely used media for adherent and suspension cells, respectively, but many lines will prefer a medium tailored to their cell type.
One key supplement is Fetal Bovine Serum (FBS), which is broadly added to many culture systems. If your cultures prefer serum, follow the recommended percentage guidelines for established lines, or optimize for in-house lines, and remain consistent with your serum source. A common question is whether or not to heat inactivate the serum. Heat inactivation halts the complement system, and so this step is typically only required in immunology studies to avoid confounding variables.
-
Do your cells feel comfortable?
When working with a cell culturing system, the environment we place our cells in can have the biggest impact on overall cell viability. Be aware of factors such as temperature and CO2 drift; even small variations in incubator calibration can have big impacts on your cells. Without regular maintenance and calibration, the temperature and CO2 readings on your incubator display may be incorrect. And it’s easy to forget about the water pan, but keeping it filled and the chamber completely humidified is essential to cell maintenance, or else we risk evaporation in our cultures.
For suspension cells, ensure rocking or rotation of any platforms are set to an appropriate speed. Recommended speeds often cover a range, and optimizing this variable can make all the difference for the viability of your cultures.
-
How friendly are your cells?
Too few or too many cells can easily stress a culture and affect behavior. Some cells can grow very independently and may prefer lower seeding densities during maintenance periods. Others require close contact with each other in order to thrive. While a certain amount of estimation can be acceptable for some lines, other cultures require strict adherence to confluency minimums and maximums. Failure to understand the density needs of your cells risks stressing a culture and altering the performance.
Also consider the status of your cultures. Primary cells and immortalized cells often need to be treated differently, even for the same cell type. Perhaps your established line needs to be passed three times a week, but your primary cultures need more time to rest and grow.
Also consider the duration of your experiment; if your seeding density gave you 90% confluence after 24 hours, be sure to scale the cell number so as not to overcrowd and stress the experimental culture if changing to a longer time point.
If an experiment does go awry, it can be difficult to pinpoint where the culture issues may have started. For this reason, be diligent about recording passage numbers and freeze/thaw dates, which can be instrumental to track cell behavior trends.
-
How adherent are your cells?
The polystyrene plastic of flasks, plates, and dishes is normally uncharged. This neutral state causes the plastic to be hydrophobic, or repellent to water and general binding of molecules. Plastic can become “tissue culture treated”, which is achieved using plasma gas or corona gas to generate a charge. These methods change the structure of the plastic slightly and it becomes negatively charged, thus hydrophilic, which attracts the charged membranes of cells and promotes cell adhesion.
While many cells are naturally adaptive to adhere to and thrive on glass or treated plastic surfaces, some lines require a little extra TLC. In these cases, coatings such as gelatin, collagen, or Poly-D-Lysine may be added to culture surfaces. These compounds simulate the extracellular matrix and can help cells adhere more securely.
Additionally, we can consider here the tools we are using. The removal and addition of media and reagents can be disruptive to cells. When, for example, we are changing media on our cultures, we may try to perform the task quickly in order to prevent cells from drying out. Though unintentional on our end, the force of the media dispensing from the serological pipet may be too much for some sensitive cultures and can even cause them to detach from their growing surface. To easily prevent this issue, use a pipet controller that slowly dispense media at a consistent pace. The Low and Variable Speed models of the ali-Q™ 2 are designed to gently deliver media and solutions to your cells without watching the lines on the pipet.
-
What home do your cells prefer?

The term “tissue culture treatment” may sound suitable for any culture type, but there is an important distinction to be made between adherent and suspension cultures. While adherent cultures normally require a surface which promotes cell attachment, namely glass or tissue culture-treated plastics, suspension cultures do not have such requirements. Additionally, the use of non-treated surfaces may be critical for certain cultures. For example, non-treated or ultra-low bind plates are recommended when culturing monocytes in order to prevent them from adhering to a surface and differentiating into macrophages.
-
What volumes are being transferred?
Consider your experimental plan and evaluate what volumes you are transferring and how you are transferring them. Practically any experiment can be boiled down to the precise transfer of liquids, yet how we perform these transfers is often overshadowed by what we are transferring. Selecting the most appropriate plastic consumables can help mitigate the risk of contamination.
While pipette tips are sterile, your pipettor very likely is not, even with thorough cleaning and exposure to UV in the biosafety cabinet. One size of tool does not fit every job, and for this reason you should have a variety of tube options available, such as 10mL centrifuge tubes. The shorter length of this tube decreases the chance of your pipettor coming into contact with the inside of the tube, thus decreasing the risk of contamination. Another option is extended length pipette tips.
-
Are your cells hosting an uninvited guest?
Difficult to detect and even more difficult to eliminate, mycoplasma can infect cultures and wreak havoc on your experiments. If your baseline readouts seem to be drifting, it’s very possible that mycoplasma is to blame. Unlike a bacterial or fungal infection, mycoplasma is not easily visualized in cultures. While 0.22μm filters are suitable for capturing the vast majority of microbes, mycoplasma is small enough to pass through these pores. In order to ensure that no uninvited guests are entering your cultures, filter your media and reagents using 0.1μm membranes.
-
We are all human, but we don’t have to measure inaccurately like one!
We all have our moments and make errors, it’s an unavoidable part of being human. “Did I just pipette 20μl or 50μl? The serological pipet dripped, did each dish get exactly 2mL?” We can mitigate many of these common issues with tools designed to help us work more effectively.
Fixed volume, color-coded MLA pipettes are an ideal option for protocols that rely on set values, virtually eliminating errors related to inputting the incorrect volume on the pipette. MLAs are excellent for accuracy and traceability, there is no possibility of “dial drift” occurring while actively pipetting. Along with the MLAs, the Ovation® pipettes feature options to help avoid input errors with the ability to program volume presets in the electronic models. Ovation pipettes are uniquely designed to reduce fatigue and manual strain, common problems that affect proper technique and lead to irreproducible results. If you experience pain from daily pipetting in your hands, arms and body – you may be compromising your experiment.
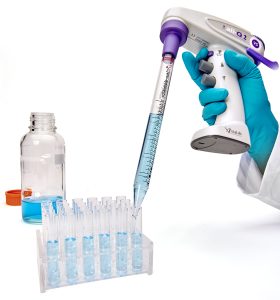
When it comes to larger volumes, the ali-Q 2 accurately measures out 0.5 – 5mL aliquots, which takes the guesswork and tedium out of serial pipetting media or other solutions. Paired with the no drip tip of the Wobble-not™ serological pipets, you can rest assured that you delivered an accurate volume.
Culturing cells is not easy, there are many factors which can disrupt their growth and affect their behavior. Healthy cells are the cornerstone of successful experiments. Treat your cells well, they will thank you!
